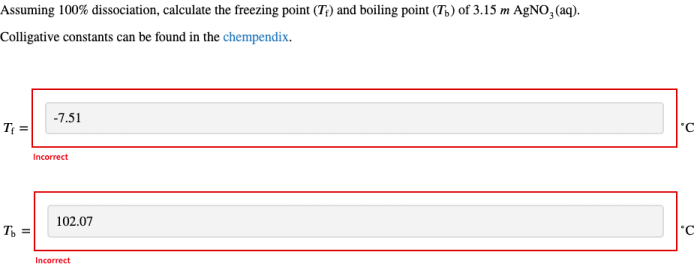
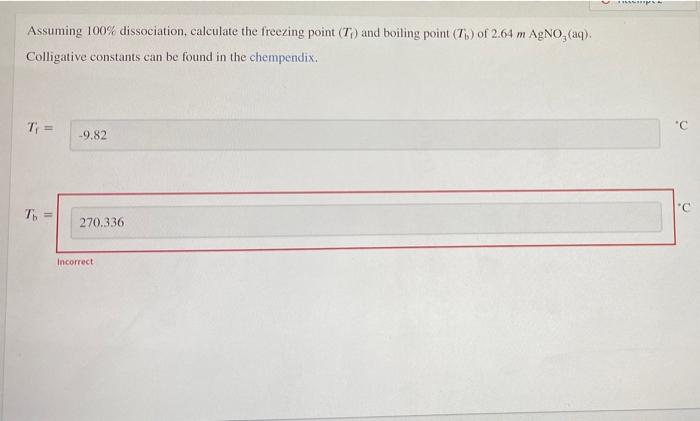
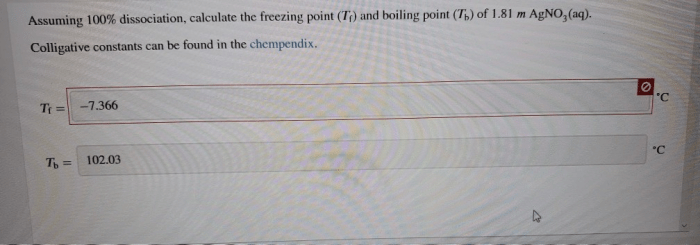
Assuming 100 dissociation calculate the freezing point – Assuming 100% dissociation, calculating the freezing point depression of a solution involves understanding the colligative properties of solutions, the concept of freezing point depression, and the dissociation of solutes. This article explores the relationship between these concepts and provides a step-by-step guide to calculating freezing point depression for dissociated solutions.
Freezing point depression is a colligative property that describes the decrease in the freezing point of a solvent when a solute is dissolved in it. The extent of freezing point depression depends on the number of solute particles present in the solution, and for dissociated solutes, the degree of dissociation must be considered.
Colligative Properties of Solutions: Assuming 100 Dissociation Calculate The Freezing Point
Colligative properties are properties of solutions that depend on the number of solute particles in solution, not on the nature of the solute particles. The four colligative properties are vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure.
Freezing Point Depression
Freezing point depression is the decrease in the freezing point of a solvent when a solute is added to it. The freezing point depression is directly proportional to the molality of the solution.
The equation for calculating freezing point depression is:
ΔTf= Kf× m
where:
- Δ Tfis the freezing point depression
- Kfis the freezing point depression constant of the solvent
- mis the molality of the solution
Dissociation, Assuming 100 dissociation calculate the freezing point
Dissociation is the process by which a compound separates into ions in solution. The extent of dissociation depends on the nature of the compound and the solvent.
For example, NaCl dissociates completely in water to form Na +and Cl –ions.
Calculating Freezing Point Depression for Dissociated Solutions
When a compound dissociates in solution, the number of solute particles increases. This results in a greater freezing point depression than would be expected for a non-dissociating compound.
To calculate the freezing point depression of a solution containing a dissociated solute, the van’t Hoff factor ( i) must be used. The van’t Hoff factor is a measure of the number of ions that are produced when a compound dissociates.
The equation for calculating freezing point depression for a dissociated solution is:
ΔTf= Kf× m× i
where:
- Δ Tfis the freezing point depression
- Kfis the freezing point depression constant of the solvent
- mis the molality of the solution
- iis the van’t Hoff factor
Applications of Freezing Point Depression
Freezing point depression is used in a variety of applications, including:
- Determining the molecular weight of a solute
- Making antifreeze
- Preserving food
- Measuring the salinity of water
User Queries
What is the relationship between freezing point depression and the concentration of a solution?
Freezing point depression is directly proportional to the concentration of the solution. The more concentrated the solution, the greater the freezing point depression.
How does dissociation affect the freezing point depression of a solution?
Dissociation increases the number of solute particles in a solution, which leads to a greater freezing point depression.
What are the assumptions made when calculating the freezing point depression of a dissociated solution?
The assumptions include complete dissociation of the solute, negligible interactions between solute particles, and an ideal solution behavior.