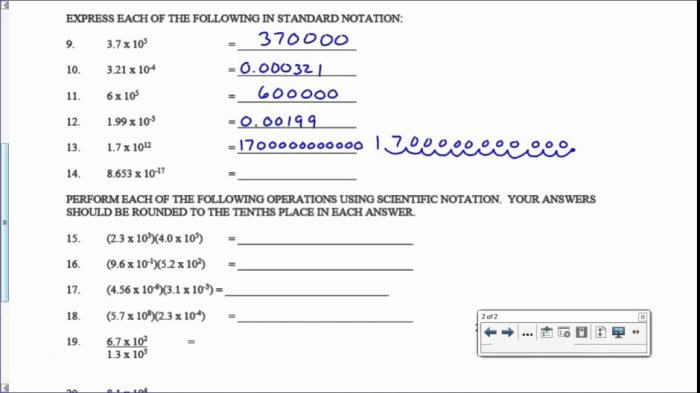
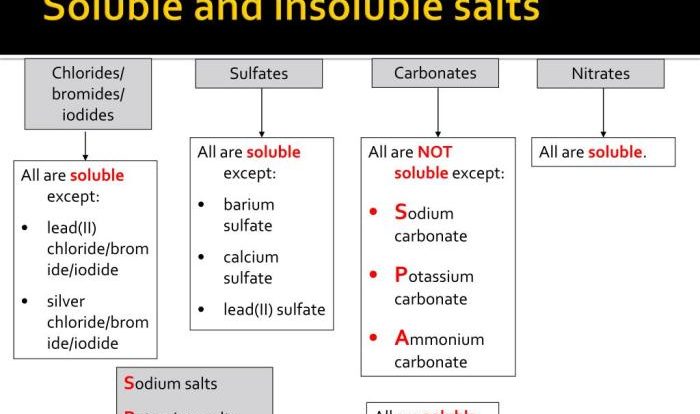
Electron configuration orbital notation worksheet – Embark on a journey into the fascinating realm of electron configuration and orbital notation with our meticulously crafted worksheet. This invaluable resource empowers you to delve into the intricacies of electron arrangements and unravel the mysteries of atomic structure.
Prepare to grasp the fundamental principles of electron configuration, unravel the complexities of orbital notation, and master the art of representing electron distributions with precision.
Electron Configuration Orbital Notation
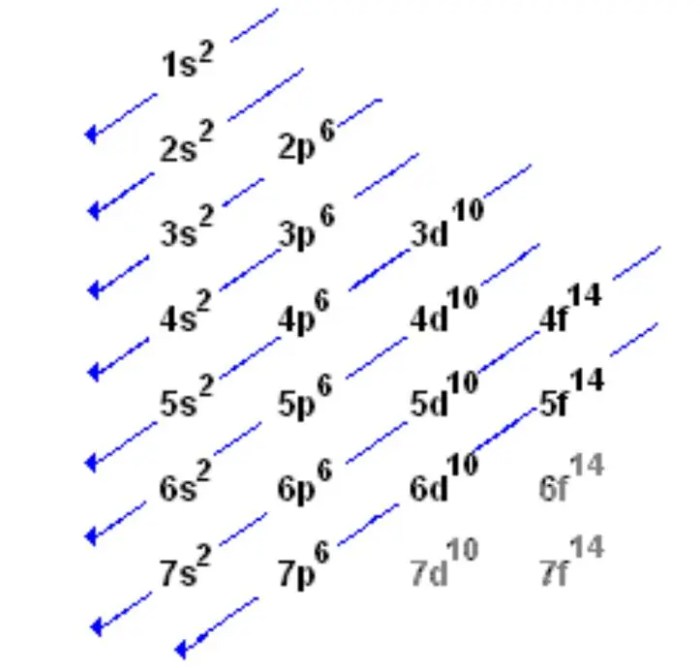
Electron configuration orbital notation is a method for representing the distribution of electrons in an atom’s orbitals. It provides detailed information about the arrangement of electrons in different energy levels and sublevels within an atom.
Orbital Notation
Orbital notation uses symbols to represent the different types of orbitals (s, p, d, f) and the number of electrons occupying each orbital. The s orbital is spherical, the p orbitals are dumbbell-shaped, the d orbitals have more complex shapes, and the f orbitals are even more complex.
- s orbital: 2 electrons
- p orbital: 6 electrons (3 pairs)
- d orbital: 10 electrons (5 pairs)
- f orbital: 14 electrons (7 pairs)
The shapes and orientations of the different orbitals are as follows:
s orbital:Spherical p orbitals:Dumbbell-shaped, oriented along the x, y, and z axes d orbitals:More complex shapes, including cloverleaf and double dumbbell shapes f orbitals:Even more complex shapes
Electron Configuration in Orbital Notation, Electron configuration orbital notation worksheet
To write an electron configuration in orbital notation, start by determining the total number of electrons in the atom. Then, fill the orbitals in the following order: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, and so on.
For example, the electron configuration of carbon is 1s 22s 22p 2. This means that carbon has two electrons in the 1s orbital, two electrons in the 2s orbital, and two electrons in the 2p orbital.
| Element | Atomic Number | Electron Configuration |
|---|---|---|
| Hydrogen | 1 | 1s1 |
| Helium | 2 | 1s2 |
| Lithium | 3 | 1s2 2s1 |
| Beryllium | 4 | 1s2 2s2 |
| Boron | 5 | 1s2 2s2 2p1 |
Key Questions Answered: Electron Configuration Orbital Notation Worksheet
What is the significance of electron configuration?
Electron configuration plays a pivotal role in determining the chemical properties of elements, as it governs their reactivity, bonding behavior, and position on the periodic table.
How does orbital notation differ from electron configuration?
While electron configuration provides a concise representation of the number of electrons in each energy level, orbital notation offers a more detailed visualization of their spatial distribution within specific orbitals.
What are the benefits of using worksheets for practicing electron configuration?
Worksheets provide a structured and interactive environment for practicing electron configuration and orbital notation, allowing students to test their understanding, identify areas for improvement, and reinforce their knowledge.