Step into the captivating realm of chemistry with our in-depth exploration of soluble and insoluble salts lab answers. Embark on a scientific adventure where we unravel the mysteries of solubility, its governing factors, and the practical applications that shape our world.
Prepare to be enlightened as we delve into the intricacies of salt behavior, empowering you with a profound understanding of this fundamental chemical concept.
Solubility of Salts
Solubility refers to the ability of a substance to dissolve in a solvent, forming a homogeneous mixture. In the case of salts, solubility depends on several factors, including temperature, pressure, and the nature of the salt and solvent.
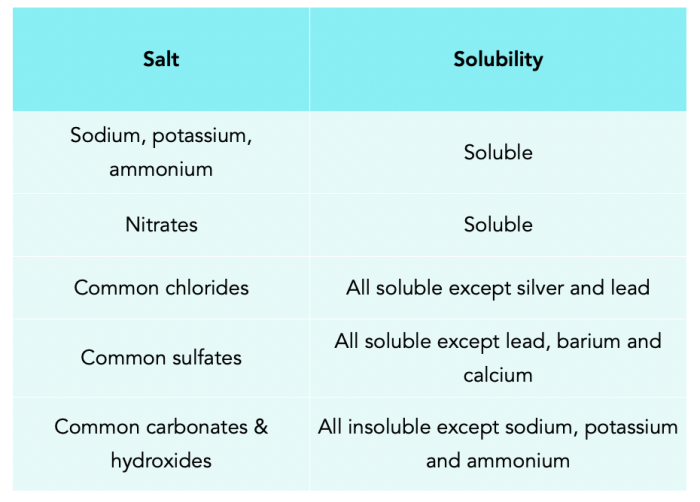
Soluble salts dissolve readily in water, forming clear solutions. Examples of soluble salts include sodium chloride (NaCl), potassium chloride (KCl), and calcium chloride (CaCl 2). Insoluble salts, on the other hand, do not dissolve appreciably in water and form suspensions or precipitates.
Examples of insoluble salts include barium sulfate (BaSO 4), calcium carbonate (CaCO 3), and silver chloride (AgCl).
Laboratory Procedures: Soluble And Insoluble Salts Lab Answers
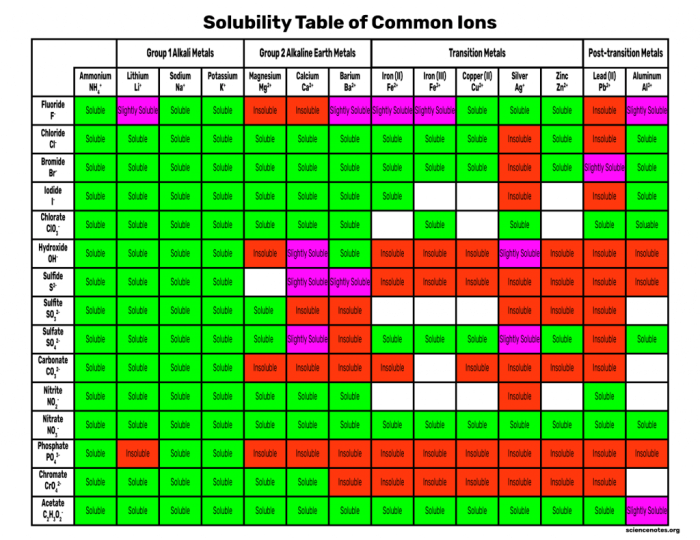
Experimental Setup
The experiment involves testing the solubility of different salts in water. The setup includes:
- Test tubes or small beakers
- Samples of different salts
- Distilled water
- Stirring rod or spatula
Steps
- Add a small amount of salt to a test tube or beaker.
- Add a few milliliters of distilled water and stir.
- Observe whether the salt dissolves completely or forms a precipitate.
- Repeat steps 1-3 for different salts.
Recording and Interpreting Observations
Record the observations in a table, noting whether each salt is soluble or insoluble. The solubility can be classified as:
- Very soluble: Dissolves completely
- Moderately soluble: Dissolves partially
- Slightly soluble: Dissolves to a small extent
- Insoluble: Does not dissolve
Data Analysis
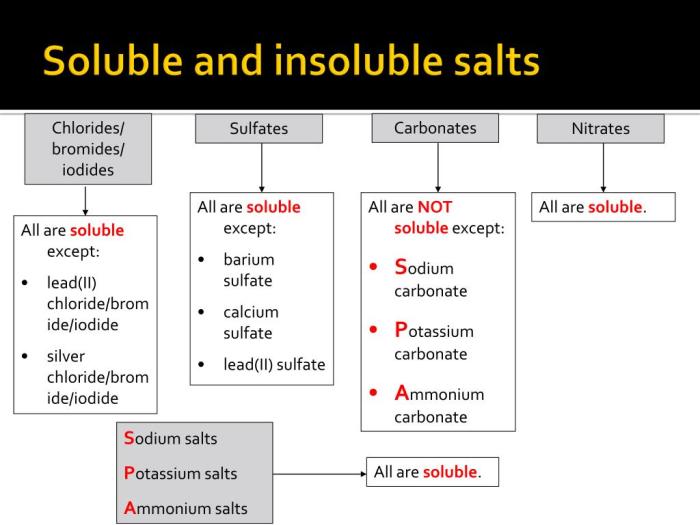
Solubility Table
Organize the solubility data in an HTML table:
| Salt | Solubility |
|---|---|
| Sodium chloride (NaCl) | Very soluble |
| Potassium chloride (KCl) | Very soluble |
| Calcium chloride (CaCl2) | Very soluble |
| Barium sulfate (BaSO4) | Insoluble |
| Calcium carbonate (CaCO3) | Insoluble |
| Silver chloride (AgCl) | Insoluble |
Patterns and Trends
Identify patterns and trends in the solubility data:
- Salts with similar cations or anions tend to have similar solubilities.
- The solubility of salts generally increases with temperature.
- The solubility of salts can be affected by the pH of the solution.
Salt Structure and Solubility
The solubility of a salt is influenced by its structure:
- Ionic salts, composed of positively charged cations and negatively charged anions, tend to be more soluble than covalent salts.
- Salts with smaller ions are generally more soluble than salts with larger ions.
- Salts with a higher charge density are generally more soluble than salts with a lower charge density.
Applications of Salt Solubility
Everyday Life
- Dissolving salt in water to create a saltwater solution for cooking or preserving food.
- Using salt to remove ice from roads during winter.
- Adding salt to water for swimming pools to prevent algae growth.
Industrial Applications
- Producing chlorine and sodium hydroxide through the electrolysis of brine (sodium chloride solution).
- Manufacturing paper and textiles using salt solutions.
- Using salt in the production of glass and ceramics.
Environmental Implications, Soluble and insoluble salts lab answers
- Saltwater intrusion in coastal areas due to rising sea levels.
- Soil salinization due to irrigation with saline water.
- Eutrophication of water bodies due to excess salt runoff from agricultural fertilizers.
Safety Considerations
Handle salts with care, as some can be toxic or corrosive.
- Wear gloves and eye protection when working with salts.
- Avoid inhaling or ingesting salts.
- Dispose of salt solutions properly according to local regulations.
Clarifying Questions
What is the key factor that determines the solubility of a salt?
The polarity of the solvent is a crucial factor in determining the solubility of a salt. Polar solvents, such as water, tend to dissolve ionic compounds like salts more readily than nonpolar solvents.
How can we differentiate between soluble and insoluble salts?
A simple laboratory test can be performed to distinguish between soluble and insoluble salts. When a salt is added to a solvent and dissolves completely, it is considered soluble. If the salt remains suspended in the solvent or settles at the bottom, it is considered insoluble.
What are some real-world applications of salt solubility?
Salt solubility plays a vital role in various industries. In the food industry, it is used to preserve and flavor foods. In the pharmaceutical industry, it is employed in the production of medicines and drug delivery systems. Additionally, salt solubility is crucial in water treatment processes and environmental remediation.