Are ch3ch2ch2ch2ch3 and h2o miscible – At the heart of this inquiry lies the intriguing question of miscibility between CH3CH2CH2CH2CH3 and H2O. Miscibility, the ability of two liquids to form a homogeneous mixture, unveils a captivating realm of intermolecular interactions and practical applications.
Delving into the depths of this topic, we will explore the fundamental principles of miscibility, meticulously dissect the properties of CH3CH2CH2CH2CH3 and H2O, and meticulously design an experiment to experimentally determine their miscibility. Furthermore, we will illuminate the diverse applications of miscibility, showcasing its profound impact on various scientific disciplines and everyday life.
Miscibility of Ch3ch2ch2ch2ch3 and H2o
Miscibility refers to the ability of two liquids to form a homogeneous mixture when combined. It is determined by several factors, including molecular structure, polarity, and temperature.
Ch3ch2ch2ch2ch3 (pentane) and h2o (water) are immiscible liquids. This is because pentane is a nonpolar hydrocarbon, while water is a polar molecule. Nonpolar molecules have an even distribution of electrons, while polar molecules have an uneven distribution of electrons. This difference in polarity prevents pentane and water from mixing.
Properties of Ch3ch2ch2ch2ch3 and H2o: Are Ch3ch2ch2ch2ch3 And H2o Miscible
Ch3ch2ch2ch2ch3 (Pentane)
- Nonpolar hydrocarbon
- Low boiling point (-36.1 °C)
- Low density (0.626 g/cm3)
- Insoluble in water
- Flammable
H2o (Water)
- Polar molecule
- High boiling point (100 °C)
- High density (1 g/cm3)
- Soluble in many polar solvents
- Non-flammable
Comparison of Properties, Are ch3ch2ch2ch2ch3 and h2o miscible
| Property | Ch3ch2ch2ch2ch3 | H2o |
|---|---|---|
| Polarity | Nonpolar | Polar |
| Boiling point | -36.1 °C | 100 °C |
| Density | 0.626 g/cm3 | 1 g/cm3 |
| Solubility in water | Insoluble | Soluble |
| Flammability | Flammable | Non-flammable |
Experimental Determination of Miscibility

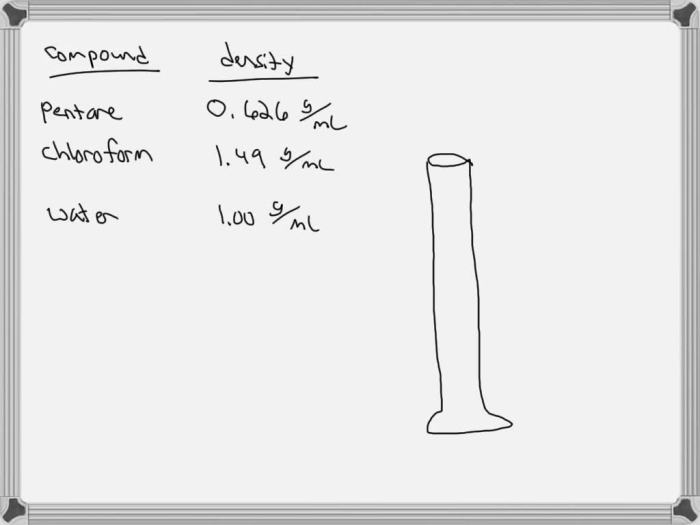
To determine the miscibility of ch3ch2ch2ch2ch3 and h2o, a simple experiment can be conducted.
Materials:
- Ch3ch2ch2ch2ch3
- H2o
- Graduated cylinder
- Funnel
Procedure:
- Add 10 ml of ch3ch2ch2ch2ch3 to a graduated cylinder.
- Add 10 ml of h2o to the graduated cylinder.
- Stopper the graduated cylinder and shake vigorously.
- Observe the mixture.
Results:
The two liquids will form two distinct layers. The ch3ch2ch2ch2ch3 layer will be on top, and the h2o layer will be on the bottom. This indicates that ch3ch2ch2ch2ch3 and h2o are immiscible.
Applications of Miscibility
Miscibility has a wide range of applications in various fields, including:
- Chemistry:Miscibility is used to separate and purify liquids through techniques such as distillation and extraction.
- Pharmaceuticals:Miscibility is important in the development of drugs and drug delivery systems.
- Cosmetics:Miscibility is used to create emulsions and other cosmetic products.
- Food industry:Miscibility is used to create emulsions, sauces, and other food products.
- Environmental science:Miscibility is used to study the behavior of pollutants and other chemicals in the environment.
User Queries
Are CH3CH2CH2CH2CH3 and H2O polar or nonpolar?
CH3CH2CH2CH2CH3 is nonpolar, while H2O is polar.
What factors influence the miscibility of liquids?
Factors such as polarity, molecular size, and intermolecular forces play a crucial role in determining miscibility.